
The reason for this behaviour is that protein folding is severely affected by heat and, therefore, some chaperones act to repair the potential damage caused by misfolding. Many chaperones are heat shock proteins, that is, proteins expressed in response to elevated temperatures or other cellular stresses. 2 Nomenclature and examples of bacterial and archeal chaperones.It is for this reason that many chaperones, but by no means all, are also heat shock proteins because the tendency to aggregate increases as proteins are denatured by stress.
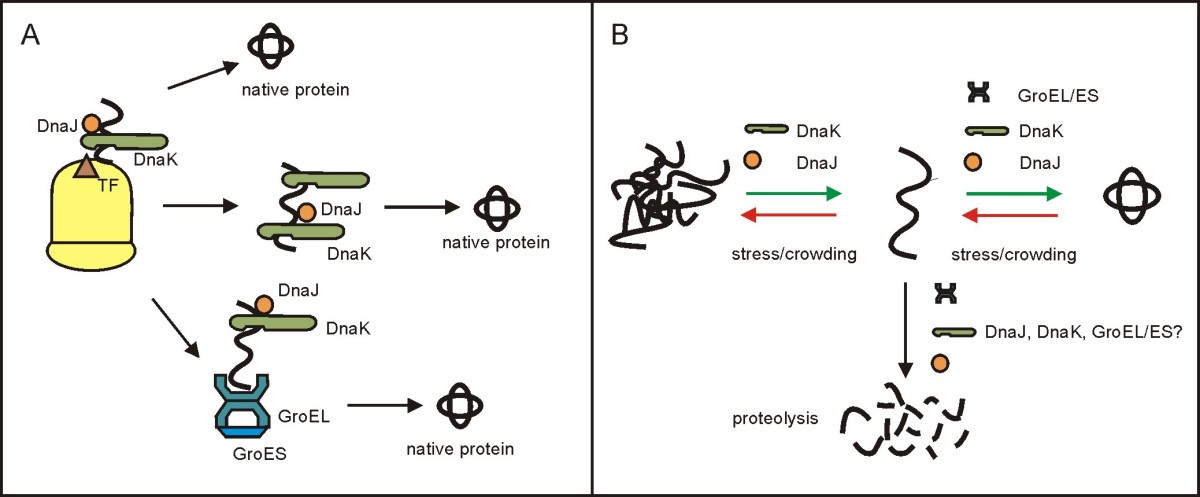
One major function of chaperones is to prevent both newly synthesised polypeptide chains and assembled subunits from aggregating into nonfunctional structures. The first protein to be called a chaperone assists the assembly of nucleosomes from folded histones and DNA and such assembly chaperones, especially in the nucleus, are concerned with the assembly of folded subunits into oligomeric structures.Ĭhaperones do not convey steric information required for proteins to fold: thus statements of the form `chaperones fold proteins` are misleading. The common perception that chaperones are primarily concerned with protein folding is incorrect. In molecular biology, chaperones are proteins that assist the non-covalent folding/unfolding and the assembly/disassembly of other macromolecular structures, but do not occur in these structures when the latter are performing their normal biological functions. For the person who accompanies another during social situations, see chaperon.


 0 kommentar(er)
0 kommentar(er)
